What is pH?
We often associate pH ranges and values with chemical reactions as a chemical can be highly effective at a specific pH value. For example, Water corrosion and softening are controlled by adjusting the pH values. But what is pH? pH is short for potential of Hydrogen. pH is a measure of Hydrogen Ion activity in a liquid. To understand more clearly about pH and its measurement, you need to first understand the molecular structure of Water. Water exists as continually associating and disassociating Hydrogen Ions and Hydroxide Ions in any i.e. H+ and OH– respectively. H2O = H+ + OH– So, in any aqueous solution, in addition to the usual Hydrogen and Hydroxide ions, there will also be normal H2O and H3O+. But the important value is the concentration of the Hydrogen Ions. An important observation is that the product of concentration of Hydrogen Ions and concentration of Hydroxide Ions is constant in any aqueous solution. This product, is known as Dissociation Constant of Water and is represented by the symbol KW. Mathematically, KW = [H+] [OH–], and the square brackets indicate the molar concentration of the Hydrogen Ions and Hydroxide Ions. KW is dependent on temperature. For example, at 250C, water contains approximately 1 x 10-7 moles per liter of hydrogen ions and 1 x 10-7 moles per liter of hydroxide ions. So, the value of KW at 250C is 1 x 10-14 and at 350C, it is approximately 1.47 x 10-14.
Acid, Base and Neutral
When either acids or bases are dissolved in water, they tend to alter the concentration of Hydrogen Ions (H+) and Hydroxide Ions (OH–). When acids are dissolved, they increase the H+ ions concentration and decrease the OH– ions concentration. This is because, the final product of the molar concentration of H+ and OH– ions must be constant. When bases are dissolved, they have an opposite effect i.e. they increase the concentration of OH– ions and decrease the concentration of H+ ions. Let us understand this by taking a look at an example. Suppose, an acid is dissolved in water at 250C. It will increase the concentration of Hydrogen ions to 1 x 10-4 moles per liter. Since the product of [H+] and [OH–] must be equal to 1 x 10-14, the concentration of Hydroxide Ions can be calculated as 1 x 10-10 moles per liter. pH is an alternative way of expressing the concentration of Hydrogen Ions. Mathematically, pH is the negative logarithm of molar concentration of Hydrogen Ions [H+]. pH = -log [H+] If we apply this formula to the above example, where the concentration of Hydrogen Ions [H+] is 1 x 10-4, the value of pH is 4. When discussing about acids and bases, the term neutral often comes up. A solution is said to be neutral if the concentration of Hydrogen Ions [H+] is equal to the concentration of Hydroxide Ions [OH–]. So, at 250C, a neutral solution will have a pH value of 7. If pH is less than 7, then the solution is acidic in nature and if the pH is greater than 7, then the solution is basic in nature. A neutral solution at 350C will have a pH value of about 6.92.
How pH is Measured?
Determining the value of pH by first measuring the concentration of Hydrogen Ions [H+] and then calculating pH using the above log equation is not the best way. There is usually a simple setup used involving pH electrodes to measure the pH of a solution. The setup consists of an electrochemical cell. One half of the cell consists of a Measuring Electrode or an Indicating Electrode, which is made up of thin Sodium Ion Selective Glass. The potential of this Measuring Electrode is directly proportional to the pH of the liquid. The other half of the cell consists of a Reference Electrode with potential independent of the pH of the liquid. The purpose of the reference electrode is to provide electrical continuity in order to measure the potential.
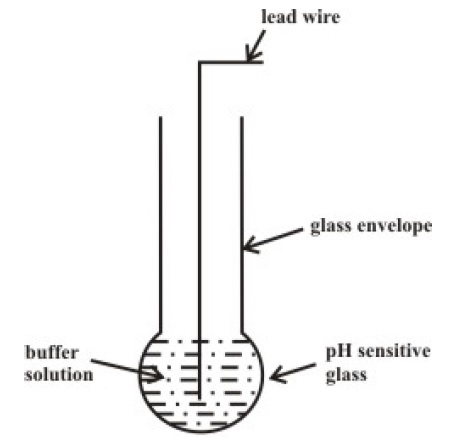
Indicating Electrode (Measuring Electrode)
Following is the simple representation of a measuring electrode. As mentioned earlier, it consists of a Glass Bulb with a Buffer Solution and a lead wire. When this glass bulb is dipped in an aqueous solution, an electric potential is developed across the surface of the glass. This potential is related to the concentration of H+ Ions with a sensitivity of 59.2 mv/pH at 250C. Coming to the Buffer, it is a solution with stable pH value and precisely known pH value. The buffer helps in calibrating the Electrodes as each measuring electrode will have a different response to pH.
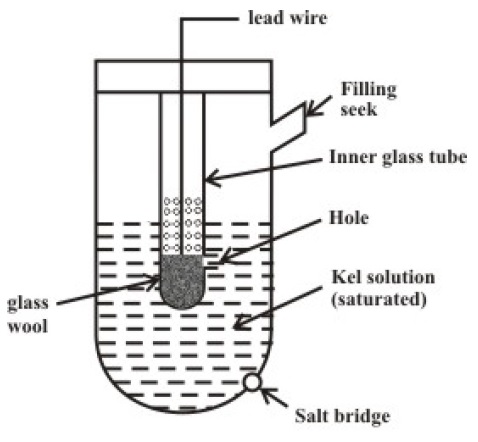
Reference Electrode
As mentioned earlier, the Reference Electrode is to provide electrical continuity in order to measure the potential. When both the Measuring Electrode and Reference Electrode are placed in the solution whose pH is to be measured, the potential is measured across the two lead wires. Since the reference electrode doesn’t directly participate in the measurement of the pH of the solution, the potential developed at the reference electrode must have the following characteristics.
It should be independent of Concentration of H+ Ions. It should be independent of temperature. It should not change with time.
Based on the above characteristics, there are two commonly used reference electrodes.
Mercury Mercurous Chloride (Calomel) Silver-Silver Chloride
Measuring pH
As mentioned earlier, the sensitivity of the measuring electrode is 59.2 mv/pH at 250C. In order to measure the pH Voltage, a special measuring circuit is required. This is because, the internal resistance of the probe used for measuring pH Voltage is very high, usually 100 MΩ to 1000 MΩ. The reason for such high internal resistance is because it must be minimum 10 times the resistance of the electrode. Finally, a FET based amplifier or an Op-Amp circuit is used to amplify the pH Voltage.
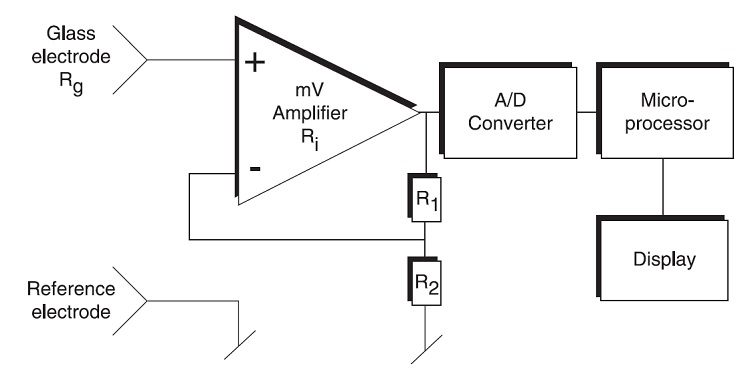
pH Sensor and pH Meter
A pH Sensor is a device that measures the pH of an aqueous solution in laboratory and industrial environments. A pH Sensor is a complete setup consisting of Measuring Glass Electrode, Reference Electrode, Amplifier, Analog to Digital Converter, Microcontroller and a Display. This entire setup with sensor and measuring units along with the display units is sometimes referred to as pH Meters. The following image shows a simplified block diagram of a typical pH Sensor or pH Meter.
Influence of Temperature on pH Sensor
The characteristics of Buffer solution and the pH Electrodes of a pH Sensor are severely dependent on the temperature. Ideally, the sample liquid, the buffer solution and the pH Electrodes of the pH Sensor should all be maintained at the same temperature. Practically, a temperature difference of 20C to 50C is acceptable in most pH Sensors.
Applications of pH Sensor
The measurement of pH of a solution is very important in several fields like Biology, Agriculture, Food and Nutrition, Forest and Environmental Studies, Oceans, Medicine and Chemistry etc. Some of the common applications of a pH Sensor are mentioned below:
Beer and Wine Fermentation Sea water Monitoring Mud and Soil Analysis Standard Lab Measurements High pH Solutions Natural Water Analysis Blood Analysis Food Stability and Preservation
Comment * Name * Email * Website
Δ





![]()